Fuel Cell Catalyst: Less is More
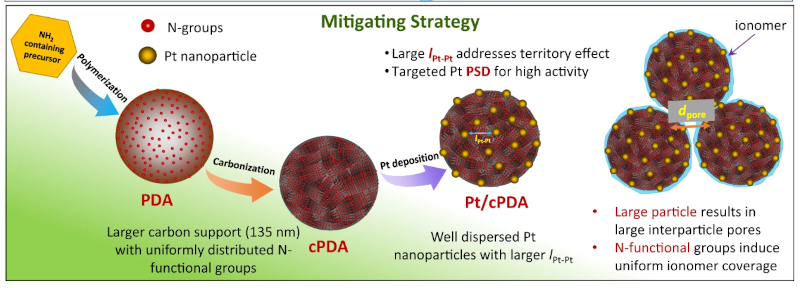
A fuel cell is almost like a battery that has replenishable fuel. Instead of charging a battery with an electric current, you recharge a fuel cell with something like hydrogen or you simply consume it from a tank much as an internal combustion engine consumes gasoline. However, fuel cells usually use a catalyst — it isn’t consumed in the reaction, but it is necessary and many fuel cells use platinum as a catalyst which is expensive. But what if you could use less catalyst and get a better result? That’s what researchers in Canada and the US are claiming in a recent paper. The key isn’t how much catalyst they are using, but rather the shape of the catalyst.
Of course, everyone wants to use less of the expensive catalyst but polymer electrolyte fuel cells have had a particular problem where reducing the amount of catalyst used causes a disproportionate drop in cell performance. This new approach uses spherical catalyst support that improves the distribution and utilization of the catalyst.
One of the biggest problems with hydrogen fuel cells, of course, remains the storage of the hydrogen. Toyota has a proposed answer for that. We’ve also seen proposals for storing it in a paste form.
We are always surprised we don’t see more homebrew activity in the area of practical fuel cells. Sure, there’s RACHEL the drone. The last big push we saw was back in 2015, but those projects have either disappeared or suspended further work.
Post a Comment