Big Chemistry: Synthetic Oil

For as long as I’ve been driving, I’ve been changing oil. Longer than that, actually — before I even got my license, I did a lot of the maintenance and repair work on the family car. It seemed natural to do it back then, and it continues today, despite the fact that it would probably be cheaper overall to farm the job out. I keep doing it mainly because I like keeping in touch with what’s going on with my cars.
Oil changes require supplies, but the last few times I made the trip to BigBoxMart I came back empty-handed. I don’t know whether it’s one of the seemingly endless supply chain problems or something else, but the aisle that usually has an abundance of oil was severely understocked. And what was there was mostly synthetic oil, which I’ve never tried before.
I’ve resisted the move to synthetic motor oil because it just seemed like a gimmick to relieve me of more of my hard-earned money than necessary. But now that it seems like I might have little choice but to use synthetic oil, I thought I’d do what normally do: look into the details of synthetic oils, and share what I’ve found with all of you.
Old Mechanic’s Tales
Right up front, I’ll say that there appears to be a lot of “folklore” about motor oils in general and synthetics in particular, and a lot of strong feelings among people for whom cars are more than mere transportation. So it’s easy to find videos and blog posts that insist that synthetics are a gift from the lubrication gods, and ones that declaim against synthetics in the strongest possible terms. And of course, each camp looks at the other as heretics, whose lubrication predilections are sure to lead them into a pit of automotive despair and suffering. Such is our polarized world, I suppose.
While I don’t really want to choose sides in the motor oil wars, I definitely do not want to do anything that could potentially damage the carefully tended engines of my cars. Having never run synthetics, I did feel a little due diligence was in order: is it possible for synthetics to cause damage to older engines that have only run traditional oils?
The short answer is: probably not. When synthetic oils first came out, their chemistry was not entirely compatible with current engine technology. Specifically, the early ester-based synthetics caused problems with engine seals containing polyester resins. Those days are long gone, as both engine seal technology and synthetic oil formulations have improved. Modern synthetics have been tested to be compatible with all sorts of materials commonly used in engine seals, like nitrile, silicone, polyacrylates, and fluoroelastomers like Viton. Oils that bear the proper testing certifications have been shown not to cause excessive swelling or shrinking, hardening, or reduction in strength in seal material when exposed to synthetic oil.
So basically, if you’ve got an engine made any time in the last 30 years or so, and you use a synthetic oil that matches the manufacturer’s recommendations, you should be fine.
All About the Base
But what exactly is it about synthetic motor oil that makes it synthetic? And how does it differ from traditional motor oil? As it turns out, there’s less difference than you’d think between the two oils, but the way that they differ is pretty interesting, and the differences revealed the world of lubrication engineering in a way that I never really appreciated before.
All motor oils, traditional or synthetic, are highly engineered products that contain a bewildering array of additives, each with a specific job. However, motor oils all start with a base oil, which falls into one of five broad groups, based on properties such as sulfur content, viscosity, and the amount of saturated hydrocarbons it contains — more on that later:
| API BASE OIL CATEGORIES | |||||
|---|---|---|---|---|---|
| Base Oil Category | Sulfur (%) | Saturates (%) | Viscosity Index | ||
| Mineral | Group I (solvent refined) | > 0.03 | and/or | < 90 | 80 to 120 |
| Group II (hydrotreated) | < 0.03 | and | > 90 | 80 to 120 | |
| Group III (hydrocracked) | < 0.03 | and | > 90 | > 120 | |
| Synthetic | Group IV | Poly-α-olefin (PAO) lubricants | |||
| Group V | All other base oils | ||||
Group I, II, and III base oils are all derived from crude oil, and the methods used to refine the crude feedstock into lighter fractions suitable for engine oil largely determine the amount of sulfur in the base oil, as well as the concentration of unsaturated hydrocarbon compounds in it. Saturated hydrocarbons are ones where each carbon in the backbone of the polymer is fully populated with hydrogens; in other words, saturated compounds have no carbon-carbon double bonds. This is important because unsaturated bonds are potential sites for oxidation, which can damage the properties of the base oil.
Synthetic, Yes, But…
So since the base oils for traditional mineral oils come straight from the ground in the form of crude oil, surely that must mean that synthetic oil avoids the stain of association with fossil fuels. As it turns out, that’s not the case. If there’s one thing we’ve learned from this “Big Chemistry” series, it’s that almost everything we use in daily life comes, in whole or in part, from petrochemicals. And it’s the same with synthetic oil.
Group IV base oils are almost exclusively poly-α-olefins, or PAOs. Like plastics, PAOs are synthetic polymers, but rather than having massively long and intricately cross-liked sidechains, PAOs are mostly a small number of short sidechains linked together. But, plastics and PAOs have a lot in common, in terms of starting materials and the processes needed to create them.
The starting point for most polymers is natural gas. The primary compound in natural gas is methane (CH4), the simplest hydrocarbon possible and a member of the alkane family, which has a backbone of fully saturated carbons. But most natural gas deposits also have a significant concentration of more complicated alkanes, such as the four-carbon butane, three-carbon propane, and two-carbon ethane. Ethane is the starting point for much polymer chemistry, and can be isolated from raw natural gas by selective condensation.
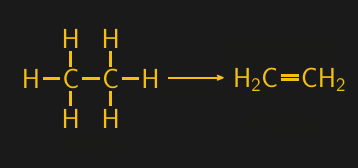
Ethane gas can be converted into ethylene gas by steam cracking, which uses high temperatures and pressures to break down saturated hydrocarbons and introduce carbon-carbon double bonds. Hydrocarbons with at least one double bond are referred to as alkenes, and the alkene version of ethane is called ethylene. And ethylene, with its chemically reactive and centrally located double bond, is an extremely useful building block.
The next step along the way to synthetic oil is making longer chain alkenes from ethylene. The chemical process used for this, the Ziegler process, is complex and beyond the scope of this article – and honestly, beyond my understanding. But suffice it to say that it uses an aluminum-containing organic catalyst to stick multiple ethylene compounds together. The Ziegler process allows fine control over the oligomerization reaction, and can produce alkenes of specific lengths. When the carbon backbone has ten carbons with a single double bond, it’s called 1-decene.
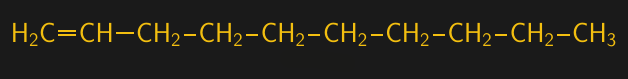
Alkenes, which are also known as olefins, are characterized by where along the carbon backbone their double bond is located. When the double bond is located next to the first, or alpha, carbon, the resulting molecule is called an alpha-olefin, or α-olefin. 1-decene is the most common α-olefin used in the production of poly-α-olefin, which is accomplished by reacting 1-decene with a catalyst of boron trifluoride (BF3) and either water or an alcohol. This reaction targets the vulnerable double bond on the α-carbon of 1-decene, linking it to the α-carbon on another 1-decene molecule.
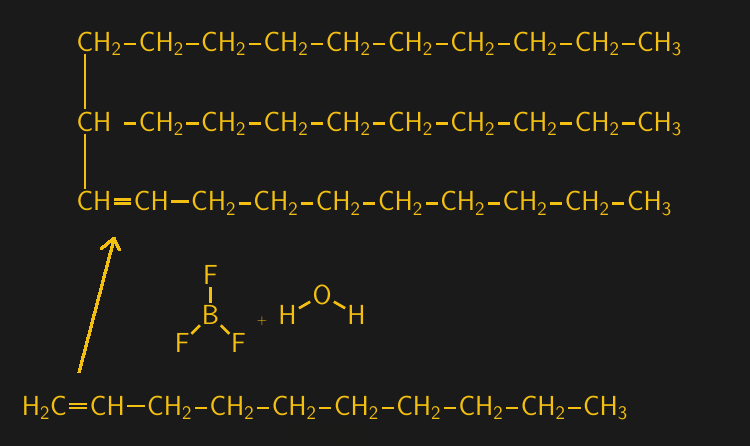
Unlike in plastics, the polymerization reaction is only allowed to go for a few rounds, yielding small oligomers of 1-decene. Five or six rounds are typical, resulting in a PAO that is completely devoid of sulfur compounds and unsaturated bonds (the remaining double bond from the last reaction is saturated by reacting the PAO with hydrogen gas under pressure. That removes the last possible site for oxidation, making for a stable PAO base oil.
Almost Done
PAOs are manufactured in huge volumes and shipped to blending facilities where the base oils are mixed together to achieve the proper viscosity. Additives such as detergents, dispersants, anti-foaming agents, and anti-wear agents are added to meet the specifications of the final product.
While the name may be misleading, in many cases synthetic motor oils hold the edge over their mineral oil counterparts. When making a motor oil from a mineral base oil, it’s nearly impossible to avoid potentially performance-limiting problems, especially oxidation sites. On the other hand, synthetic oils can almost entirely avoid introducing those oxidation sites, leading to a final product that lasts longer and performs better throughout its life.
I don’t know if I’ll ever be able to bring myself to go 25,000 miles without changing the oil, which synthetic manufacturers often advertise as being possible, but now I know what’s behind that claim, and that the cost of synthetics is at least justified by the amount of engineering that goes into them.
Post a Comment